Introduction: Bone marrow biopsies (BMB) are usually performed before/after therapy to confirm complete response (CR) in follicular lymphoma (FL) patients. Up to 60% of patients may have bone marrow infiltration, that can change Ann Arbor stage, assigning the highest disease stage. Also, BMB infiltration is included in several prognostic indexes such as FLIPI2 and PRIMA-PI. Persistence of bone marrow disease after treatment is associated with worse outcome.
Aim: The main objective is to establish whether BMB adds value in assessing response or predicting progression-free survival (PFS) and overall survival (OS) in previously untreated FL subjects from a large, Spanish unicentric retrospective cohort.
Results: From 2001-2022 we recorded data from 260 newly diagnosed FL grade 1-3A from Hospital 12 de Octubre.The median age at diagnosis was 64 years (range 27-90) and 34 patients (13%) had grade 3A FL. Most patients (67%) had advanced Ann-Arbor stage and 45,4% presented bone marrow infiltration (111/244). 104 (40%) patients were considered suitable for a watchful waiting policy; of them, 52 (50%) never required systemic treatment during follow-. 132 patients (61% of those receiving treatment) were treated with R-CHOP or R-CVP, 44 (21%) received R-bendamustine and 20 (10%) rituximab monotherapy. In 114 patients (55%) PET/CT was done at the moment of diagnosis and to evaluate the response after treatment. The other were evaluated by CT.
Basal bone marrow biopsy was essential in the diagnostic of 3 patients that only presented FL in BM. In addition, BMB upgraded disease stage in 9 (3.7%) that presented <3 nodal areas involved, without other extranodal infiltration.
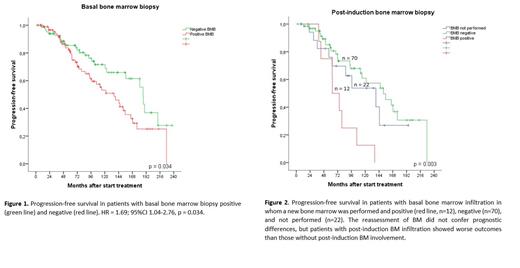
Basal BMB positive was predictive of worse PFS and OS in univariate analysis (HR = 1.69; 95%CI 1.04-2.76 and HR = 1.98; 95%CI 1.10-3.60, respectively). Ten-years PFS was 72% in patients without BM involvement vs 51% in patients with BM infiltration (HR= 1.7, p=0.034) ( Figure 1) and, Ten-years OS was 82% vs 62%, respectively.
In 82 of 104 patients (79%) with basal BM infiltration, a BMB was performed after finishing induction treatment. In the other 22 patients BMB was not performed because of evidence of progression (n=10) or because of unknown causes (n=12). 12 (15%) of 82 patients evaluated remained BMB positive by histology.
Globally, and independent of the image response assessment, the reassessment of BM did not confer prognostic differences in patients in whom it was performed and in those who were not (p= 0.92). Nevertheless, patients with post-induction BM infiltration (n=12) showed worse outcomes than those without post-induction BM involvement (n=70) (HR=3.9, 95CI 1.8-8.5) ( Figure 2).
Only 3 of 59 treated patients (5%) had positive baseline BMB, CR on imaging, and subsequent positive BMB, and therefore that affected response assessment. All 3 patients were evaluated by CT because they received treatment in 2004-2007. Of subjects with CR on imaging (n = 73), PFS and OS were not significantly different among those with negative BMB to confirm CR (n = 59) versus those without repeat BMB (n = 11); PFS: HR=1.12, 95%CI 0.38-3.31, p = 0.83; OS: HR=1.66 95%CI 0.46-6.07, p = 0.44)
Conclusion: In a real-life study of newly diagnosed FL we conclude that BMB adds little value in the basal evaluation; with only 4.% of patients who were thought to have early stage disease before bone marrow evaluation. Besides, despite BM infiltration confers prognostic value, to date there are no differences in the management of these patients based on this finding.
In relation to response assessment, BMB adds little value to response assessment. Only 3 patients (1.4%) in our series presented CR on imaging and BM infiltration postinduction. Moreover, none of them was evaluated by PET.
With this data, we strongly recommend performing diagnostic BMB in FL in scenarios in which it may change management including confirmation of limited stage and assessment of cytopenias. Prospective studies are necessary to see its value during follow- up. Nevertheless, with new techniques as LiqBio MRD, removing this study from reassessment might be an option that would reduce patient discomfort and resource utilization.
Disclosures
Grande:AbbVie: Other: Advisory Board. Ayala:Novartis: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Astellas: Speakers Bureau; BMS: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal